Introduction
While radiotherapy is the established standard of care for limited stage, grade 1-2 follicular lymphoma (FL), the management of localized grade 3A (FL3A) disease remains controversial. We reviewed the outcomes of grade 3A patients treated with definitive RT, systemic therapy (ST) alone or combined modality therapy (CMT) of RT plus ST.
Methods
We analyzed a single institution database to identify newly-diagnosed, FL3A patients with Ann Arbor stage I/II disease treated between 2000-2021. All cases were reviewed by the Hematopathology staff at the time of diagnosis. Patients were stratified by definitive treatment modality including RT monotherapy, CMT, or ST. Best post-RT imaging response was evaluated using Lugano criteria for all by CT, and by PET if available. Rate of complete response (CR), overall survival (OS), progression-free survival (PFS) and time to out-of-field progression were compared between the three treatment modalities. Progression-free survival (PFS) and overall survival (OS) were calculated using Kaplan Meier from first day of treatment. Time to out-of-field progression was estimated via cumulative incidence function from treatment start date with competing events death without out-of-field progression of disease (PD) and in-field PD. Univariable Cox regression was used to assess possible clinicodemographic associations with PFS.
Results
84 patients (median age 62, 38% male) were analyzed with median follow-up of 6.9 years from treatment. Patients were stage I (73%) or II (27%) and 92% were initially PET staged at diagnosis. 29 (35%) had extranodal disease. The most common site of extranodal disease was the head and neck (13% of patients). The median (IQR) baseline maximum SUV and maximum lesion size prior to treatment were 9.8 (5.4, 14.0) and 3.2 (2.0, 4.5) cm respectively, and did not differ between treatment groups.
RT was the most commonly utilized treatment modality (n=48, 57%), followed by CMT (n=21, 25%), then ST (n=15, 18%). For RT/CMT patients the planned RT dose was most commonly 36 Gy (32%, range 4-40Gy). 2 patients (3%) received a very low dose program beginning with 4 Gy. Of the CMT/ST patients the most common regimen was 3-4 cycles of R-CHOP (53%). 3 patients (8%) received Rituximab alone. All ST patients were diagnosed after 2010. The ST subset had the highest proportion of patients with stage II disease (p<0.001), while CMT patients had the highest proportion of female patients (p=0.03) and grade 3 disease not further characterized (p=0.002).
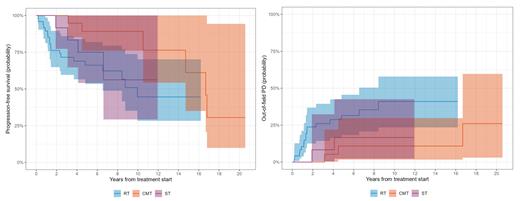
The rates of CR post-treatment were 88% for RT, 100% for CMT, and 87% for ST. Of the 22 patients who relapsed, only 1 suffered an infield relapse alone (4.5%) with the rest experiencing distant relapse (95.5%). The 5-year cumulative incidence of out-of-field progression was 32% (95%CI 18-46%) for RT, 11% (95%CI 1.7-30%) for CMT, and 17% (95%CI 2.3-43%) for ST.
There were factors significantly associated with PFS univariably including the treatment modality (CMT vs. RT HR 0.30, 95% CI 0.10-0.91, p=0.03), and presence of low-grade FL component in the biopsy (HR 3.33, 95% CI 1.43-7.78, p=0.005). Age, sex, stage, extranodal status, Ki67 score, max pre-treatment size, and max pre-treatment SUV were not associated with PFS. The probability of transformation to DLBCL was 4.2% (95% CI 1.1-11%) at 5 years after treatment. Overall survival was excellent for all treatment modalities with median OS of 18 years for CMT, and not reached for RT or ST.
Conclusion
To our knowledge this is the largest study of the outcomes of early stage FL3A after therapy. CMT had the highest rate of CR in our cohort, and was associated with a statistically significantly lower hazard of PFS compared to RT. However, OS is excellent regardless of treatment modality suggesting all may be appropriate options.
Disclosures
Zelenetz:Abbvie: Research Funding; BMS: Consultancy, Honoraria; None other than mutual funds (401K): Current equity holder in publicly-traded company; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; SAB: Membership on an entity's Board of Directors or advisory committees; Lymphoma Research Foundation: Membership on an entity's Board of Directors or advisory committees; MEI Pharma Inc: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Janssen Pharmaceuticals: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria. Salles:ATB Therapeutics: Consultancy; BeiGene: Consultancy; BMS/Celgene: Consultancy; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; AbbVie: Consultancy, Honoraria; Debiopharm: Consultancy; Genmab: Consultancy; Incyte: Consultancy; Janssen: Consultancy, Research Funding; Kite/Gilead: Consultancy; Loxo/Lilly: Consultancy; Merck: Consultancy, Honoraria; Molecular Partners: Consultancy; Novartis: Consultancy; Nurix: Consultancy; Orna: Consultancy; Ipsen: Consultancy, Research Funding; Nordic Nanovector: Consultancy; Owkin: Current holder of stock options in a privately-held company; EPIZYME: Consultancy. Yahalom:Convergent R.N.R Ltd.: Other: Provision of Services (uncompensated). Imber:GT Medical Technologies: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal